BRACKETING APPROACH
The cleaning process of multi product use equipment are subjected to requirements of cleaning validation. The validation effort could be huge. In order to minimize the amount of validation required, a worst case approach of for the validation can be used.
Cleaning procedures for products or process which are very similar do not need to be individually validated. A single validation study under consideration of worst case can then be carried out which takes account of relevant criteria used for worst case selection.
The bracketing approach may be considered acceptable for similar products and/or equipment’s provided appropriate justification based on sound and scientific rationale is given.
Company should demonstrate the objective of bracketing and its scientific rationale for its worst case rating of the substances in the cleaning validation programme.
Approach:
By means of bracketing procedure the substances/ products/ equipment’s are grouped and then sub grouped as applicable.
A worst case rating procedure is used to select the worst case in each group/sub group as applicable.
Validation of worst case situation takes place. However it is of utmost important that a documented scientific rational for chosen worst case exist.
Grouping by Equipment Train:
For example if a multipurpose site has manufacturing number of organic substances by using number of equipment trains as given below.
Train A – 9 Substances can be produced which have same cleaning procedure
Train B – 9 Substances can be produced which have same cleaning procedure
Train C – 8 Substances can be produced with two different cleaning procedures. Out of 8 substances 4 substances have cleaning procedure-A, and other 4 have different cleaning procedure-B
Train D – 8 Substances can be produced which have same cleaning procedure
Train E – 10 Substances can be produced which have same cleaning procedure.
Train F –11 Substances can be produced Out of 11 substances 6 substances have cleaning procedure-C, and other 5 have different cleaning procedure-D.
With no bracketing and worst case rating cleaning validation studies required for each of 55 substances.
The substances to be grouped first based on equipment train. Hence 6 groups will be formed as per above data. Then the groups to be sub grouped based on cleaning procedure. Hence 2 sub-groups will be formed in each Train C & Train F groups.
Finally the company would have 8 groups for cleaning validation purpose as follows
Train A – 1 Group
Train B – 1 Group
Train C – 2 Group
Train D – 1 Group
Train E – 1 Group
Train F – 2 Groups
Once the product groups have been established the next step is determined the so-called ‘worst case’ representative of each group and cleaning validation of the same.
By using bracketing approach we validated only 8 products out of 55 products.
Grouping by Substances:
Substances can be grouped as follows
Produce in the same train substances with the same cleaning procedure.
Produce in the same train substances with very low therapeutic dose and/or low batch sizes. (Then sub groups to be formed based on cleaning process)
Produce in the same train substances with very high therapeutic dose and/or large batch sizes. (Then sub groups to be formed based on cleaning process).
Produce in the same train substances with very low ADE. (Then sub groups to be formed based on cleaning process).
Produce in the same train substances with very High ADE. (Then sub groups to be formed based on cleaning process).
Once the product groups have been established the next step is determined the so-called ‘worst case’ representative of each group and cleaning validation of the same.
Grouping by Product:
1. The common basis for grouping is by product. The grouping is usually based on the formulations or dosage form of the product. When this approach is used products are divided in to groups according to the dosage form and then according to formulation.
For example
A company might have 10 tableted products, 6 ointment products and 4 liquid products. In this case the first evaluation would be that the products fall naturally in to 3 broad groups.
However if 6 of the tableted products were manufactured by wet granulation process, whereas 4 of the products were manufactured by a dry, direct compression method this would be a basis for subdividing the tablet in to 2 sub-groups. Likewise if 2 of liquid products were suspensions and other 2 liquid product were true solutions. This will also create 2 subgroups for this group.
The company would have 5 groups of products for cleaning validation purpose.
Tableted products – 2 groups
Ointment products – 1 group
Liquid products – 2 groups.
Once the product groups have been established the next step is determined the so-called ‘worst case’ representative of each group.
2. Another example is would be a group composed of several products of similar potency. In this case the worst case selection might be based on the basis of solubility.
3. Third example might be group composed of several products having same API and differing only in concentration of API. In this it would be reasonable to select product having highest concentration as worst case.
It is unlikely that single worst case product could apply to entire line of products having significantly different formulation and dosage forms.
The substance / Product which does not fall within bracketing approach must be validated individually.
WORST CASE RATING
Worst case rating will generally depend on following points.
a) Hardest to clean, Experience from production
b) Solubility in used solvent
c) Lowest acceptable daily exposure
d) Lowest therapeutic dose
Hardest to clean, Experience from production:
One criterion which can be used is, experience from production with regard to how difficult a substance is to clean out. This study is recommended to be in the form of interviews with operators and supervisors.
Difficulty of cleaning could be rated according to the three categories suggested below.
Category:
1 = Easy
2 = Medium
3 = Difficult
Solubility in used solvent:
Solubility rating should be carried out as follows.
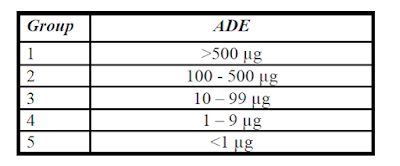
Acceptable daily exposure (ADE):
The acceptable daily exposure (ADE) defines a limit at which patient may be exposed every day for a life time with acceptable risk related to adverse health effects.
ADE rating should be carried out as follows.
If ADE data are not available, other pharmacological (dose), OEL or toxicity data LD50 may be used.
Therapeutic dose:
Rating based on therapeutic dose can be given as follows.
If dose data are not available, other pharmacological (dose), OEL or toxicity data LD50 may be used.
Rating Procedure:
The worst case rating can be executed according to an issued protocol in which the methods and procedures for rating will be identified. And a formal rating matrix has been filled as follows.
For example if a group has formed from 9 substances (Esubstance, Fsubstance, Csubstance, Lsubstance, Osubstance, Msubstance, Psubstance, Rsubstance and Tsubstance) which can produce from same equipment train. Out 9 substances 6 substances have one cleaning procedure where as other 3 have different cleaning procedure.
All categories are introduced as column in matrix to identify worst case based on rating.
For the products in this train two cleaning procedures (Class 1 & Class III) are used.
Therefore two groups have to be validated.
The worst case product (for the validation study) for class III is Osubstance (Solubility 2 and hardest to clean is 2.8).
The worst case product (for the validation study) for class I is Rsubstance (Solubility 2 and hardest to clean is 2.6)
In both cases the limit should be calculated with the most active substance (ADE4) if ADE data not available the limit should be calculated with the most active substance (Therapeutic substance 4).
If limit calculated with ADE4 or therapeutic dose 4 is achievable for all products this limit can be chosen for both the groups. If limit is two low and not achievable Esubstance & Fsubstance should be considered as a separate group or produced in dedicated equipment’s.
The limit for the remaining group should be calculated with the next most active substance (i.e ADE 3 or Therapetic dose4)



Nicely explained in detail. Thanks for sharing.
ReplyDeleteVery detailed information. Thank you for sharing.
ReplyDeleteGreat Blog. You have amazingly explained it
ReplyDeletevery well explained. Thanks. It would be great if having reference of the same along with it.
ReplyDelete